jCell Therapy: A novel approach for treating blinding diseases
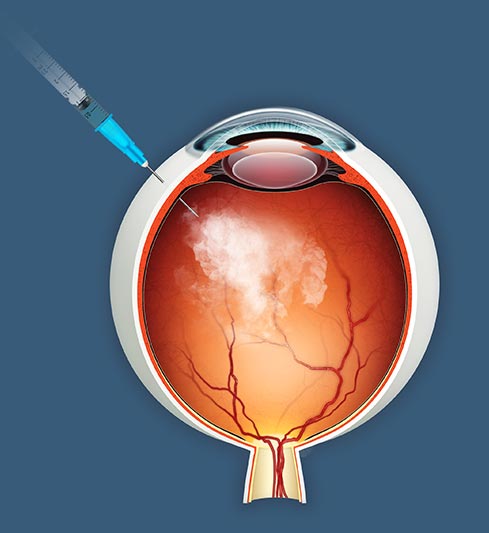 jCell is a first-in-class allogeneic cell therapy in late-stage clinical development for retinitis pigmentosa. The treatment is a minimally invasive intravitreal injection that can be performed with topical anesthetic.
jCell is a first-in-class allogeneic cell therapy in late-stage clinical development for retinitis pigmentosa. The treatment is a minimally invasive intravitreal injection that can be performed with topical anesthetic.
The principal mechanism of action is the sustained release of established neurotrophic factors that reduce photoreceptor cell death and promote function of surviving photoreceptors. jCell therapy aims to preserve vision by intervening in the disease at a time when host photoreceptors can be protected and potentially reactivated.
There are currently no FDA approved treatment options for the vast majority of patients with retinitis pigmentosa. Unlike gene therapy approaches, jCell does not target any specific genotype.
jCyte’s goal is to make jCell the first approved cell therapy to address this critical unmet medical need, and dramatically improve the lives of patients with this degenerative retinal disease.
Advantages of jCell
- Unrestricted Patient Population
- jCell does not target any specific genotype - unlike gene therapy approaches
- Scalable Platform for Multiple Ophthalmic Indications
- No Immunosuppression Required
- Expedited Regulatory Pathway
Phase 2b results presented at the American Society of Retina Specialists in July 2020, showed that jCell therapy had promising efficacy and was well tolerated in patients. Based on these encouraging results, jCyte is excited to move toward a pivotal trial as soon as possible.
Retinitis Pigmentosa: A rare disease with a sizeable population
Retinitis pigmentosa is a devastating blinding disease that begins in childhood or adolescence, which causes progressive vision loss. The syndromic forms of the disease include hearing loss and other health complications. It is a rare, genetic condition that progressively destroys the rod and cone photoreceptors in the retina, and often afflicts people in their teens, with many patients rendered legally blind by middle age.
Worldwide, an estimated 2 million people suffer from the disease, including approximately 100,000 people in the U.S., making it the leading cause of inheritable blindness.
- Occurs in approximately 1:3500 individuals worldwide
Early stage RP

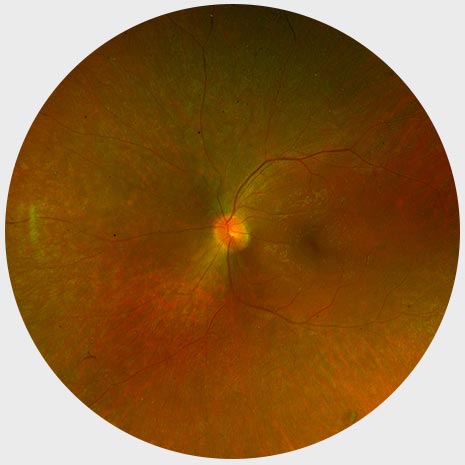
Normal Fundus
Mid-stage RP

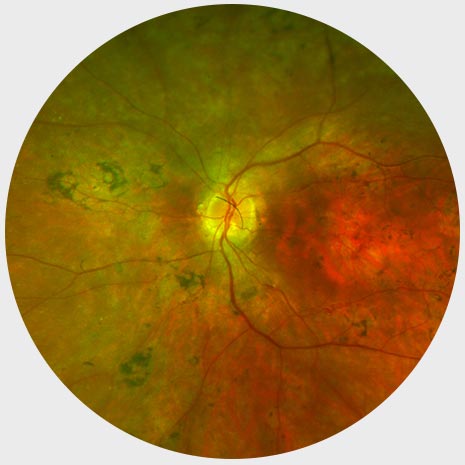
Peripheral ring of depigmentation
End stage RP

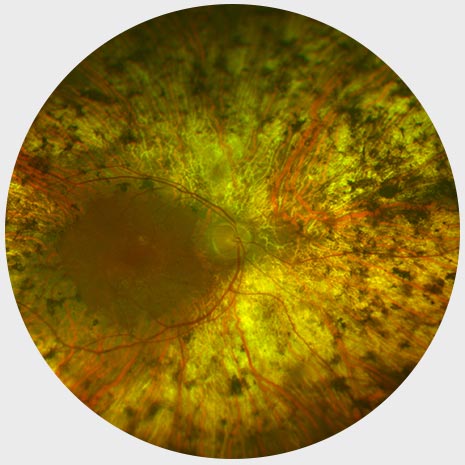
Widespread Pigment Deposits
Select Publications
Intravitreal Transplantation of Retinal Progenitor Cells Improves Outcome Measures in a Rat Model of Diabetic Retinopathy
International Journal of Molecular Science. September 2025
Safety Study of Retinal Progenitor Cells in Retinitis Pigmentosa
Frontiers in Cellular Neuroscience. August 2025
Amelioration of Photoreceptor Degeneration by Intravitreal Transplantation of Retinal Progenitor Cells in Rats
International Journal of Molecular Science. July 2024
Stem cells in clinical trials for treatment of retinal degeneration
Expert Opin Biol Ther. September 2015
Cellular manufacturing for clinical applications
Dev Ophthalmol. April 2014
Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice
PLoS One. April 2011
Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation
Biomaterials. April 2009
Expanded Access Policy
1. About jCyte
jCyte is a development-stage biotechnology company committed to advancing innovative therapies through rigorous clinical investigation and regulatory approval. Our mission is to bring safe and effective treatments to patients with with retinitis pigmentosa and other degenerative retinal disorders.
2. What is Expanded Access?
“Expanded access” (also called “compassionate use”) is a regulatory pathway that may allow a patient with a serious or immediately life-threatening condition to receive an investigational therapy outside of a clinical trial when no satisfactory alternative therapy is available and the patient cannot participate in an ongoing clinical trial. This pathway exists under regulatory frameworks such as the U.S. Food and Drug Administration (FDA) in the United States.
3. jCyte’s Position on Expanded Access
To protect patient safety, preserve scientific integrity, and support regulatory approval, jCyte believes the most appropriate way for patients to access our investigational therapies is through participation in controlled clinical trials. At this time, jCyte is not offering expanded access to its investigational therapies outside of clinical trials.
4. Clinical Trial Information
jCyte encourages patients and physicians to explore participation in our clinical trials, which represent the preferred route of access to our investigational therapies. For current information on jCyte-sponsored trials, please visit www.jcyte.com or search for “jCyte” on ClinicalTrials.gov or other regional trial registries.
For inquiries related to clinical trials or investigational therapies, please contact info@jcyte.com
5. Policy Updates
This policy may be updated from time to time without prior notice. Publication of this policy does not guarantee availability of investigational therapies for any individual or indication.

